Introduction:
Isatuximab (ISA) is an anti-CD38 monoclonal antibody that has shown benefit in patients with relapsed/refractory multiple myeloma (RRMM) in combination regimens. This study focuses on the clinical utility and tolerability of ISA based therapies in patients with ISS stage III disease.
Methods:
Literature search on PubMed, EmCare, Clinicaltrials.gov, Cochrane, and Google Scholar was performed from inception to July 1, 2020, for the use of ISA in MM patients. A total of five clinical trials, including phase Ib/II and phase III trials, were selected for a systematic review out of 109 studies (n=556 patients).
Results:
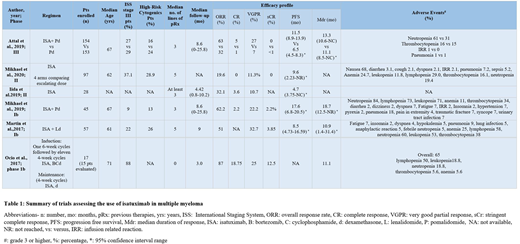
The median age was 61-71 years. The addition of ISA to pomalidomide-dexamethasone (PD) in previously treated patients was evaluated by Attal et al. (2019). International Staging System (ISS) stage III disease was present in 28% of the study population at baseline. Patients treated with ISA-PD had a meaningful increase in progression-free survival (PFS) (11.53 versus 6.47 months, P = 0.001, Hazard Ratio (HR): 0.596; 95% confidence interval (CI): 0.436-0.814. PFS benefit was maintained in subgroup analysis based on renal involvement, refractoriness to lenalidomide, and proteasome inhibitors. However, PFS benefit was not statistically significant in ISS stage III disease (HR: 0.64; CI: 0·36-1·11). Martin et al. (2017) evaluated the addition of ISA to lenalidomide and dexamethasone in 57 patients. 22% of the patients had ISS stage III disease. Mikeal et al. also included 9% and 37.1% of patients with ISS stage III MM in their phase Ib and II trials, respectively. All studies demonstrated a significant prolongation of PFS in patients refractory to lenalidomide with ISA administered at 10 mg/kg weekly/every two weeks. Iida et al. (2019) also reported statistically significant improvement in PFS with ISA monotherapy in heavily pretreated Japanese patients (PFS 4.7 months; 95% CI 3.75-NC). In patients with newly diagnosed MM, Ocio et al. (2017) showed encouraging results with an ISA based regimen in 17 patients. ISS stage III disease was present in 88% of the population. At the time of data cut-off, the overall response to treatment was 87%, and 82.4% continued to remain on treatment (see Table 1).
Conclusion:
ISA as monotherapy and in combination therapy improved surrogate endpoints without increased toxicity in patients refractory to previous lines of treatment. Limited data suggest an improvement in PFS for ISS stage III disease. Long-term results on efficacy in MM with high-risk cytogenetics are awaited.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal